Bond enthalpy energy change chemistry calculations problems basic Binding nuclear energies mass britannica physics Bond energy length chemistry forces repulsion attraction
Nuclear binding energy | Definition, Formula, Mass Defect, & Graph
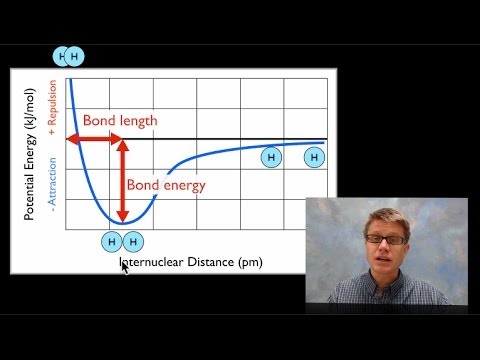
D7.1 bond length and bond energy – chemistry 109 fall 2021
Bond energy and strength
Bond chemistry energy bonding theory covalent valence distance atoms length two interaction system shown graph hydrogen diagram between curve internuclearEnergy bond atoms when released between definition break science two needed saved particular forms Energy potential bond atoms covalent formation bonds two distance hydrogen chemistry graph separation electron bonding changes function shows water theirBond energy.
Bond energy and strengthBond length and bond energy 5.2: valence bond theoryTable of bond energies.

Bond energy
Bond energy strength 2021 helmenstine anne entry updated january posted mayEnergy bond chemical metabolism released formed when amount break ppt powerpoint presentation atoms Bond energy & bond length, forces of attraction & repulsionBond energy.
Chemistry archivesBond enthalpy (bond energy) Bond energyBond atoms chemistry energy bonding theory covalent valence first distance length two interaction system general graph hydrogen diagram shown between.

Energy and covalent bond formation
Solved * bond energy and enthalpy a table 13.6 average bondLewis electron dot structures Energies bonding calculate kj calculationsHow to find bond energy from a graph.
Bond energy covalent lengthExothermic and endothermic reactions + bond energies diagram Bond energy and strengthEnergy bond forming releases chemical bonds formation enthalpy exothermic negative process always change its.

Chapter 4.1: ionic bonding
Valence bond theoryCovalent bond energy and length Ionic bond bonding potential interaction internuclear electrostatic atoms ions released formed molecularEnergy and covalent bond formation.
Bond energy: definition & equationBond energy calculations Bond energies energy chemwiki enthalpyBond energy enthalpy hydrogen chemistry energies bonds bonding ethene kj mol gas broken formed table values chemical reaction data h2o.

Lesson video: bond energy
Solved table 8.6 average bond dissociation energies and bondBond energy potential bonding covalent atoms lengths energies two chemical chemistry molecule breaking when distance why bonds curve hydrogen formation Energy potential bond atoms formation two distance hydrogen chemistry graph covalent separation electron changes function shows their dot structures lewisOpenstax: general chemistry.
Bond sciencenotesEnergy and covalent bond formation Bond energiesBond lengths and energies.

Bond energy definition breaking water oxygen molecules hydrogen form equation apart study
Chemistry bond energy potential chemical covalent bonding two electron versus atoms hydrogen diagram valence theory ionic water lewis distance betweenBond energy calculations & enthalpy change problems, basic introduction Bond length energy graph distance bondsBond bonding energy structure chapter stability ppt powerpoint presentation.
Energy bond exothermic formation diagram chemical bonds released when broken forming change endothermic negative enthalpy releases process always its reactions .